Accueil du site > Production scientifique > IRMPD spectroscopy of protonated S-nitrosocaptopril, a biologically active, synthetic amino acid
IRMPD spectroscopy of protonated S-nitrosocaptopril, a biologically active, synthetic amino acid
Date de publication: 7 novembre 2010
C. Coletti, N. Re, D. Scuderi, P. Maître, B. Chiavarino, S. Fornarini, F. Lanucara, R. K. Sinha and M. Elisa Crestoni
Phys. Chem. Chem. Phys. 12 13455-13467 (2010). DOI
Travail réalisé sur le site de l’Université Paris-Sud.
Abstract
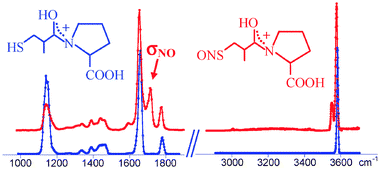
S-Nitrosocaptopril, a biologically active S-nitrosothiol, is generated as protonated species and isolated in the gas phase by electrospray ionization coupled to Fourier Transform Ion Cyclotron Resonance (FT-ICR) or ion-trap mass spectrometry. The structural and IR spectroscopic characterization of protonated S-nitrosocaptopril (SNOcapH+) is aided by the comparative study of the parent species lacking the NO feature, namely protonated captopril. The study is accomplished by methodologies based on tandem mass spectrometry, namely by energy resolved collision-induced dissociation and infrared multiple-photon dissociation (IRMPD) spectroscopy, backed by density functional theory calculations. IRMPD spectra have been obtained both in the 1000–1900 cm−1 fingerprint range, using a beamline of the infrared free electron laser (IR-FEL) at the Centre Laser Infrarouge d’Orsay (CLIO), and in the O–H and N–H stretching region (2900–3700 cm−1) using the tunable IR radiation of a tabletop parametric oscillator/amplifier (OPO/OPA) laser source. The structural features of the ion have been ascertained by comparison of the experimental IRMPD spectra with the IR transitions calculated for the lowest energy isomers. Evidence is obtained that protonation occurs at the amide carbonyl oxygen which is found to be the thermodynamically most basic site. However, SNOcapH+ is present as a thermally equilibrated mixture of low-energy structures, with a major contribution of the most stable isomer characterized by a trans relationship of the positively charged OH group with respect to the carboxylic acid functionality on the adjacent proline ring and by an anti conformation at the S–N (partial) double bond, though the energy difference with the analogous trans–syn isomer is less than 1 kJ mol−1. The highly diagnostic N–O stretching mode has been unambiguously identified, which may be regarded as an informative probe for S-nitrosation features in more complex, biologically active molecules.








