Accueil du site > Production scientifique > Short-lived intermediates (encounter complexes) in cisplatin ligand exchange elucidated by infrared ion spectroscopy
Short-lived intermediates (encounter complexes) in cisplatin ligand exchange elucidated by infrared ion spectroscopy
Date de publication: 4 octobre 2018
D. Corinti ; C. Coletti ; N. Re ; R. Paciotti ; P. Maitre ; B. Chiavarino ; ME. Crestoni ; S. Fornarini
Int. J. Mass Spectrom. 435 42917 (2019). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
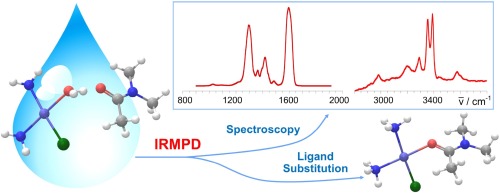
Cisplatin (cis-diamminedichloroplatinum(II), cis-[PtCl2(NH3)(2)]), widely used drug in cancer treatment, has been allowed to react with simple molecular targets (L) mimicking biological functional groups. The selected molecules (L= acetamide, dimethylacetamide, urea and thiourea) react by ligand exchange leading to cis-[PtCl(NH3)(2)(L)](+) complexes that have been assayed by ESI-MS, IRMPD spectroscopy and computations in order to characterize their structure and platination site. Formal five-coordinate complexes are also delivered by ESI, [(PtCl(NH3)(2)(H2O)(L)](+), notably absent only when L is thiourea. IRMPD spectroscopy combined with computational analysis has revealed non-covalent adducts of the reactant aqua complex with an external ligand L corresponding to cis-[PtCl(NH3)(2)(H2O)](+) center dot L, reminiscent of the Eigen-Wilkins encounter complex invoked in the ligand displacement path in solution. The complex, successfully isolated in the gas phase, undergoes ligand exchange yielding cis-[PtCl(NH3)(2)(L)](+) + H2O when activated by multiple IR photons, testifying at the same time both structure and reactivity.








