Accueil du site > Production scientifique > Role of PF-6 in the radiolytical and electrochemical degradation of propylene carbonate solutions
Role of PF-6 in the radiolytical and electrochemical degradation of propylene carbonate solutions
Date de publication: 9 juillet 2016
D. Ortiz ; I. Jimenez Gordon ; S. Legand ; V. Dauvois ; J-P. Baltaze ; J-L. Marignier ; J-F. Martin ; J. Belloni ; M. Mostafavi ; S. Le Caer
J. Power Sources 326 285-295 (2016). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
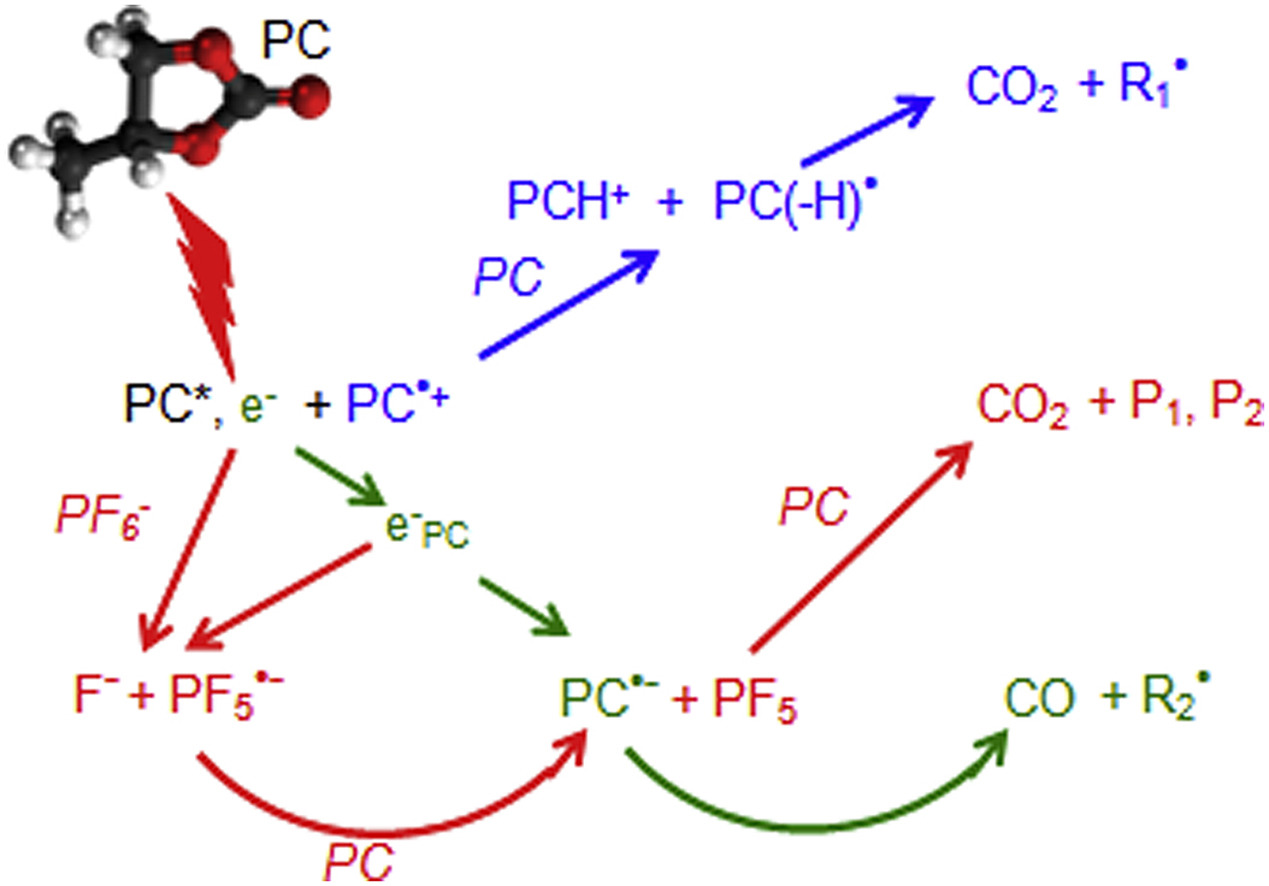
The behavior under irradiation of neat propylene carbonate (PC), a co-solvent usually used in Li-ion batteries (LIB), and also of Li salt solutions is investigated. The decomposition of neat PC is studied using radiolysis in the pulse and steady state regime and is assigned to the ultrafast formation, in the reducing channel, of the radical anion PC− by electron attachment, followed by the ring cleavage, leading to CO. In the oxidative channel, the PC(H) radical is formed, generating CO2. The CO2 and CO yields are both close to the ionization yield of PC. The CO2 and CO productions in LiClO4, LiBF4 and LiN(CF3)2(SO2)2 solutions are similar as in neat PC. In contrast, in LiPF6/PC a strong impact on PC degradation is measured with a doubling of the CO2 yield due to the high reactivity of the electron towards PF6− observed in the picosecond range. A small number of oxide phosphine molecules are detected among the various products of the irradiated solutions, suggesting that most of them, observed in carbonate mixtures used in LIBs, arise from linear rather than from cyclical molecules. The similarity between the degradation by radiolysis or electrolysis highlights the interest of radiolysis as an accelerated aging method.








