Accueil du site > Production scientifique > Insights from ion mobility-mass spectrometry, infrared spectroscopy, and molecular dynamics simulations on nicotinamide adenine dinucleotide structural dynamics : NAD(+) vs. NADH(+)
Insights from ion mobility-mass spectrometry, infrared spectroscopy, and molecular dynamics simulations on nicotinamide adenine dinucleotide structural dynamics : NAD(+) vs. NADH(+)
Date de publication: 1er février 2018
J. C. Molano-Arevalo, W. Gonzalez, K. J. D. Fouque, J. Miksovska, P. Maitre, F. Fernandez-Lima
Phys. Chem. Chem. Phys. 20 (10) 7043-7052 (2018). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
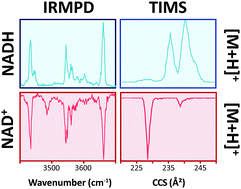
Nicotinamide adenine dinucleotide (NAD) is found in all living cells where the oxidized (NAD(+)) and reduced (NADH) forms play important roles in many enzymatic reactions. However, little is known about NAD(+) and NADH conformational changes and kinetics as a function of the cell environment. In the present work, an analytical workflow is utilized to study NAD(+) and NADH dynamics as a function of the organic content in solution using fluorescence lifetime spectroscopy and in the gas-phase using trapped ion mobility spectrometry coupled to mass spectrometry (TIMS-MS) and infrared multiple photon dissociation (IRMPD) spectroscopy. NAD solution time decay studies showed a two-component distribution, assigned to changes from a "close’’ to "open’’ conformation with the increase of the organic content. NAD gas-phase studies using nESI-TIMS-MS displayed two ion mobility bands for NAD(+) protonated and sodiated species, while four and two ion mobility bands were observed for NADH protonated and sodiated species, respectively. Changes in the mobility profiles were observed for NADH as a function of the starting solution conditions and the time after desolvation, while NAD(+) profiles showed no dependence. IRMPD spectroscopy of NAD(+) and NADH protonated species in the 800-1800 and 3200-3700 cm(-1) spectral regions showed common and signature bands between the NAD forms. Candidate structures were proposed for NAD(+) and NADH kinetically trapped intermediates of the protonated and sodiated species, based on their collision cross sections and IR profiles. Results showed that NAD(+) and NADH species exist in open, stack, and closed conformations and that the driving force for conformational dynamics is hydrogen bonding of the N-H-O and O-H-O forms with ribose rings.








