Accueil du site > Production scientifique > Structures and unimolecular chemistry of M(Pro2-H)+ (M = Mg, Ca, Sr, Ba, Mn, Fe, Co, Ni, Cu, Zn) by IRMPD spectroscopy, SORI-CID, and theoretical studies
Structures and unimolecular chemistry of M(Pro2-H)+ (M = Mg, Ca, Sr, Ba, Mn, Fe, Co, Ni, Cu, Zn) by IRMPD spectroscopy, SORI-CID, and theoretical studies
Date de publication: 10 décembre 2015
Yasaman Jami-Alahmadia, Travis D. Fridgen
Phys. Chem. Chem. Phys. 18 2023-2033 (2016). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
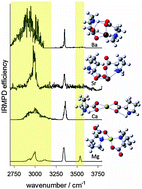
M(Pro2-H)+ complexes were electrosprayed and isolated in an FTICR cell where their unimolecular chemistries and structures were explored using SORI-CID and IRMPD spectroscopy. These experiments were augmented by computational methods such as electronic structure, simulated annealing, and atoms in molecules (AIM) calculations. The unimolecular chemistries of the larger metal cation (Ca2+, Sr2+ and Ba2+) complexes predominantly involve loss of neutral proline whereas the complexes involving the smaller Mg2+ and transition metal dications tend to lose small neutral molecules such as water and carbon dioxide. Interestingly, all complexes involving transition metal dications except for Cu(Pro2-H)+ lose H2 upon collisional or IRMPD activation. IRMPD spectroscopy shows that the intact proline in the transition metal complexes and Cu(Pro2-H)+ is predominantly canonical (charge solvated) while for the Ca2+, Sr2+, and Ba2+ complexes, proline is in its zwitterionic form. The IRMPD spectra for both Mg(Pro2-H)+ and Mn(Pro2-H)+ are concluded to have contributions from both charge-solvated and canonical structures.








