Accueil du site > Production scientifique > Distinguishing Isomeric Peptides : The Unimolecular Reactivity and Structures of (LeuPro)M+ and (ProLeu)M+ (M = Alkali Metal)
Distinguishing Isomeric Peptides : The Unimolecular Reactivity and Structures of (LeuPro)M+ and (ProLeu)M+ (M = Alkali Metal)
Date de publication: 2 décembre 2016
Yasaman Jami-Alahmadi, Bryan D. Linford, Travis D. Fridgen
J. Phys. Chem. B 120 13039-13046 (2016). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
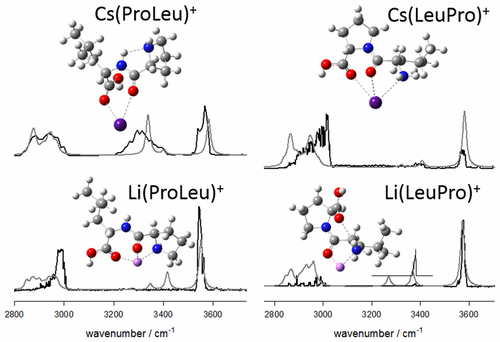
The unimolecular chemistries and structures of gas-phase (ProLeu)M+ and (LeuPro)M+ complexes when M = Li, Na, Rb, and Cs have been explored using a combination of SORI-CID, IRMPD spectroscopy, and computational methods. CID of both (LeuPro)M+ and (ProLeu)M+ showed identical fragmentation pathways and could not be differentiated. Two of the fragmentation routes of both peptides produced ions at the same nominal mass as (Pro)M+ and (Leu)M+, respectively. For the litiated peptides, experiments revealed identical IRMPD spectra for each of the m/z 122 and 138 ions coming from both peptides. Comparison with computed IR spectra identified them as the (Pro)Li+ and (Leu)Li+, and it is concluded that both zwitterionic and canonical forms of (Pro)Li+ exist in the ion population from CID of both (ProLeu)Li+ and (LeuPro)Li+. The two isomeric peptide complexes could be distinguished using IRMPD spectroscopy in both the fingerprint and the CH/NH/OH regions. The computed IR spectra for the lowest energy structures of each charge solvated complexes are consistent with the IRMPD spectra in both regions for all metal cation complexes. Through comparison between the experimental spectra, it was determined that in lithiated and sodiated ProLeu, metal cation is bound to both carbonyl oxygens and the amine nitrogen. In contrast, the larger metal cations are bound to the two carbonyls, while the amine nitrogen is hydrogen bonded to the amide hydrogen. In the lithiated and sodiated LeuPro complexes, the metal cation is bound to the amide carbonyl and the amine nitrogen while the amine nitrogen is hydrogen bonded to the carboxylic acid carbonyl. However, there is no hydrogen bond in the rubidiated and cesiated complexes ; the metal cation is bound to both carbonyl oxygens and the amine nitrogen. Details of the position of the carboxylic acid C═O stretch were especially informative in the spectroscopic confirmation of the lowest energy computed structures.








