Accueil du site > Production scientifique > Structures of bare and singly hydrated [M(Ura-H)(Ura)](+) (M = Mg, Ca, Sr, Ba) complexes in the gas phase by IRMPD spectroscopy in the fingerprint region
Structures of bare and singly hydrated [M(Ura-H)(Ura)](+) (M = Mg, Ca, Sr, Ba) complexes in the gas phase by IRMPD spectroscopy in the fingerprint region
Date de publication: 15 février 2015
B. Power, V. Haldys, J.-Y. Salpin, T.D. Fridgen
Int. J. Mass Spectrom. 378 328-335 (2015). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
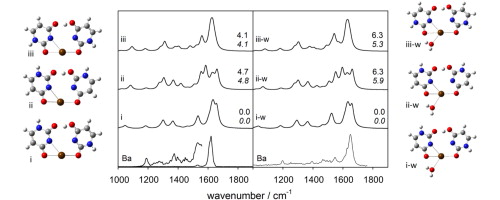
The structures of deprotonated group 2 metal dication bound uracil dimers as well as the singly hydrated dimers were explored in the gas phase using infrared multiple photon dissociation (IRMPD) spectroscopy (1000–1900 cm−1) in a Fourier transform ion cyclotron resonance mass spectrometer (FTICR-MS). The IRMPD spectra were then compared to computed IR spectra for various isomers. Calculations were performed using B3LYP with the 6–31+G(d,p) basis set for all atoms except Ba2+ and Sr2+, for which the LANL2DZ or the def2-TZVPP basis sets with relativistic core potentials were used. The lowest energy structures are those in which the one uracil is deprotonated at the N3 position while the neutral uracil is a tautomer where the N3 hydrogen is at the O4 or O2 carbonyl oxygen and the metal is tetracoordinate interacting with N3 and O4 of deprotonated uracil and either N3 and O2 or N3 and O4 of neutral uracil. In the solvated complexes, the water molecule is also coordinated to the metal ion.








