Accueil du site > Production scientifique > Gas-Phase Structure of Amyloi-β (12-28) Peptide Investigated by Infrared Spectrsoscopy, Electron Capture Dissociation and Ion Mobility Mass Spectrometry
Gas-Phase Structure of Amyloi-β (12-28) Peptide Investigated by Infrared Spectrsoscopy, Electron Capture Dissociation and Ion Mobility Mass Spectrometry
Date de publication: 17 septembre 2013
T.N. Le, J.C. Poully, F. Lecomte, N. Nieuwjaer, B. Manil, C. Desfrançois, F. Chirot, J. Lemoine, P. Dugourd, G. van der Rest, G. Grégoire
J. Am. Soc. Mass Spectrom. 24 1937-1949 (2013). DOI
Travail réalisé sur le site de l’Ecole Polytechnique.
Abstract
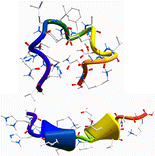
The gas-phase structures of doubly and triply protonated Amyloid-β12-28 peptides have been investigated through the combination of ion mobility (IM), electron capture dissociation (ECD) mass spectrometry, and infrared multi-photon dissociation (IRMPD) spectroscopy together with theoretical modeling. Replica-exchange molecular dynamics simulations were conducted to explore the conformational space of these protonated peptides, from which several classes of structures were found. Among the low-lying conformers, those with predicted diffusion cross-sections consistent with the ion mobility experiment were further selected and their IR spectra simulated using a hybrid quantum mechanical/semiempirical method at the ONIOM DFT/B3LYP/6-31 g(d)/AM1 level. In ECD mass spectrometry, the c/z product ion abundance (PIA) has been analyzed for the two charge states and revealed drastic differences. For the doubly protonated species, N – Cα bond cleavage occurs only on the N and C terminal parts, while a periodic distribution of PIA is clearly observed for the triply charged peptides. These PIA distributions have been rationalized by comparison with the inverse of the distances from the protonated sites to the carbonyl oxygens for the conformations suggested from IR and IM experiments. Structural assignment for the amyloid peptide is then made possible by the combination of these three experimental techniques that provide complementary information on the possible secondary structure adopted by peptides. Although globular conformations are favored for the doubly protonated peptide, incrementing the charge state leads to a conformational transition towards extended structures with 310- and α-helix motifs.








