Accueil du site > Production scientifique > Seleniranium Ions Undergo π-Ligand Exchange via an Associative Mechanism in the Gas Phase
Seleniranium Ions Undergo π-Ligand Exchange via an Associative Mechanism in the Gas Phase
Date de publication: 22 mai 2017
S. F. Lim, B. L. Harris, G. N. Khairallah, E.J. Bieske , P. Maître , G. da Silva, B. D. Adamson, M. S. Scholz , N. J. A. Coughlan, R. A. J. O’Hair, M. Rathjen, D. Stares, J. M. White
J. Org. Chem 82 6289–6297 (2017). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
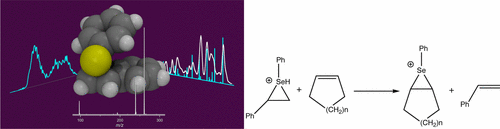
Collision-induced dissociation mass spectrometry of the ammonium ions 4a and 4b results in the formation of the seleniranium ion 5, the structure and purity of which were verified using gas-phase infrared spectroscopy coupled to mass spectrometry and gas-phase ion-mobility measurements. Ion–molecule reactions between the ion 5 (m/z = 261) and cyclopentene, cyclohexene, cycloheptene, and cyclooctene resulted in the formation of the seleniranium ions 7(m/z = 225), 6 (m/z = 239), 8 (m/z = 253), and 9 (m/z = 267), respectively. Further reaction of seleniranium 6 with cyclopentene resulted in further π-ligand exchange giving seleniranium ion 7, confirming that direct π-ligand exchange between seleniranium ion 5 and cycloalkenes occurs in the gas phase. Pseudo-first-order kinetics established relative reaction efficiencies for π-ligand exchange for cyclopentene, cyclohexene, cycloheptene. and cyclooctene as 0.20, 0.07, 0.43, and 4.32. respectively. DFT calculations at the M06/6-31+G(d) level of theory provide the following insights into the mechanism of the π-ligand exchange reactions ; the cycloalkene forms a complex with the seleniranium ion 5 with binding energies of 57 and 62 kJ/mol for cyclopentene and cyclohexene, respectively, with transition states for π-ligand exchange having barriers of 17.8 and 19.3 kJ/mol for cyclopentene and cyclohexene, respectively.








