Accueil du site > Production scientifique > Exploring the Conformational Variability in the Heme b Propionic Acid Side Chains through the Effect of a Biological Probe : A Study of the Isolated Ions
Exploring the Conformational Variability in the Heme b Propionic Acid Side Chains through the Effect of a Biological Probe : A Study of the Isolated Ions
Date de publication: 9 janvier 2015
A. De Petris, B. Chiavarino, M.E. Crestoni, C. Coletti, N. Re, S. Fornarini
J. Phys. Chem. B 119 1919-1929 (2015). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
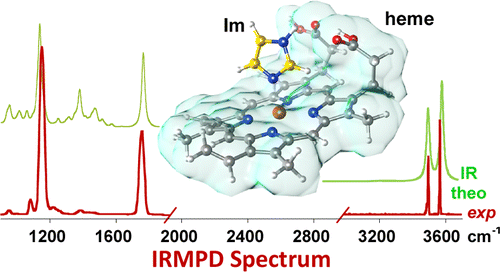
The iron(III) protoporphyrin IX complex with imidazole, a biologically relevant ligand, occupying an axial position, has been studied by infrared multiple photon dissociation (IRMPD) spectroscopy. The complex has been delivered in gas-phase by electrospray ionization (ESI), mass selected in an ion trap, and assayed by IRMPD spectroscopy in two complementary frequency regions. The fingerprint range (900–1900 cm–1) has been scanned using the Orsay free-electron laser beamline (CLIO), while the X–H (X = C,N,O) stretching region (3000–3600 cm–1) has been inspected using a tabletop IR optical parametric oscillator/amplifier (OPO/OPA) laser source. DFT calculations have been performed to obtain a comprehensive pattern of the various potential conformers yielding optimized geometries, relative thermodynamic parameters, and respective IR spectra. The comparison between the IR spectra for representative conformers and the experimental IRMPD features suggests the coexistence of two families of conformers involving different degrees of folding and hydrogen bonding between the two propionic acid functionalities on the periphery of the protoporphyrin IX macrocycle in a ratio depending on environmental conditions such as ESI solvent and temperature. The observed conformational variability of the porphyrin substituents in the naked heme–imidazole complex is consistent with the fine-tuning of the reactivity properties of this important prosthetic group by the specific surroundings in the protein core.








