Accueil du site > Production scientifique > Tandem mass spectrometry and infrared spectroscopy as a help to identify peptide oxidized residues
Tandem mass spectrometry and infrared spectroscopy as a help to identify peptide oxidized residues
Date de publication: 10 août 2015
D. Scuderi, M.T. Ignasiak, X. Serfaty, P. de Oliveira, C. Houée-Levin
Phys. Chem. Chem. Phys. 17 25998-26007 (2015). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
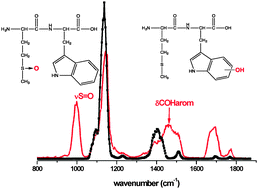
The final products obtained by the oxidation of small model peptides containing the thioether function, either methionine or S-methyl cysteine, have been characterized by tandem mass spectrometry and IR Multiple Photon Dissociation (IRMPD) spectroscopy. The modified positions have been clearly identified by the CID-MS2 fragmentation mass spectra with or without loss of sulfenic acid, as well as by the vibrational signature of the sulfoxide bond at around 1000 cm−1. The oxidation of the thioether function did not lead to the same products in these model peptides. The sulfoxide and sulfone (to a lesser extent) have been clearly identified as final products of the oxidation of S-methyl-glutathione (GS-Me). Decarboxylation or hydrogen loss are the major oxidation pathways in GS-Me, while they have not been observed in tryptophan–methionine and methionine–tryptophan (Trp–Met and Met–Trp). Interestingly, tryptophan is oxidized in the dipeptide Met–Trp, while that is not the case in the reverse sequence (Trp–Met).








