Accueil du site > Production scientifique > On the Ag + –cytosine interaction : the effect of microhydration probed by IR optical spectroscopy and density functional theory
On the Ag + –cytosine interaction : the effect of microhydration probed by IR optical spectroscopy and density functional theory
Date de publication: 29 mai 2015
M. Berdakin, V. Steinmetz, P. Maitre, G.A. Pino
Phys. Chem. Chem. Phys. 17 25915—25924 (2015). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
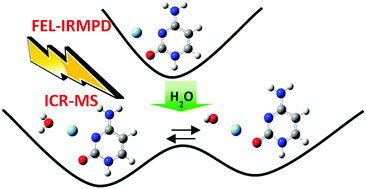
The gas-phase structures of cytosine–Ag+ [CAg]+ and cytosine–Ag+–H2O [CAg–H2O]+ complexes have been studied by mass-selected infrared multiphoton dissociation (IRMPD) spectroscopy in the 900–1800 cm−1 spectral region using the Free Electron Laser facility in Orsay (CLIO). The IRMPD experimental spectra have been compared with the calculated IR absorption spectra of the different low-lying isomers (computed at the DFT level using the B3LYP functional and the 6-311G++(d,p) basis set for C, H, N and O atoms and the Stuttgart effective core potential for Ag). For the [CAg]+ complex, only one isomer with cytosine in the keto-amino (KA) tautomeric form and Ag+ interacting simultaneously with the C(2)[double bond, length as m-dash]O(7) group and N(3) of cytosine was observed. However, the mono-hydration of the complex in the gas phase leads to the stabilization of a two quasi-isoenergetic structure of the [CAg–H2O]+ complex, in which Ag+ interacts with the O atom of the water molecule and with the N(3) or C(2)[double bond, length as m-dash]O(7) group of cytosine. The relative populations of the two isomers determined from the IRMPD kinetics plot are in good agreement with the calculated values. Comparison of these results with those of protonated cytosine [CH]+ and its mono-hydrated complex [CH–H2O]+ shows some interesting differences between H+ and Ag+. In particular, while a single water molecule catalyzes the isomerization reaction in the case of [CH–H2O]+, it is found that in the case of [CAg–H2O]+ the addition of water leads to the stabilization of two isomers separated by small energy barrier (0.05 eV).








