Accueil du site > Production scientifique > Gas-phase structure and reactivity of the keto tautomer of the deoxyguanosine radical cation
Gas-phase structure and reactivity of the keto tautomer of the deoxyguanosine radical cation
Date de publication: 5 mai 2015
L. Feketeova, B. Chan, G.N. Khairallah, V. Steinmetz, P. Maitre, L. Radom, R.A.J. O’Hair
Phys. Chem. Chem. Phys. 17 25837—25844 (2015). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
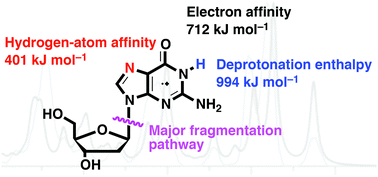
Guanine radical cations are formed upon oxidation of DNA. Deoxyguanosine (dG) is used as a model, and the gas-phase infrared (IR) spectroscopic signature and gas-phase unimolecular and bimolecular chemistry of its radical cation, dG˙+, A, which is formed via direct electrospray ionisation (ESI/MS) of a methanolic solution of Cu(NO3)2 and dG, are examined. Quantum chemistry calculations have been carried out on 28 isomers and comparisons between their calculated IR spectra and the experimentally-measured spectra suggest that A exists as the ground-state keto tautomer. Collision-induced dissociation (CID) of A proceeds via cleavage of the glycosidic bond, while its ion–molecule reactions with amine bases occur via a number of pathways including hydrogen-atom abstraction, proton transfer and adduct formation. A hidden channel, involving isomerisation of the radical cation via adduct formation, is revealed through the use of two stages of CID, with the final stage of CID showing the loss of CH2O as a major fragmentation pathway from the reformed radical cation, dG˙+. Quantum chemistry calculations on the unimolecular and bimolecular reactivity are also consistent with A being present as a ground-state keto tautomer.








